
Theoretical Insights into the Mechanism of Selective Nitrate‐to‐Ammonia Electroreduction on Single‐Atom Catalysts
Advanced Functional Materials
(
IF
19
)
Pub Date : 2020-12-21
, DOI:
10.1002/adfm.202008533
Huan Niu
1
,
Zhaofu Zhang
2
,
Xiting Wang
1
,
Xuhao Wan
1
,
Chen Shao
1
,
Yuzheng Guo
1
Affiliation
School of Electrical Engineering and Automation Wuhan University Wuhan Hubei 430072 China
Department of Engineering Cambridge University Cambridge CB2 1PZ UK
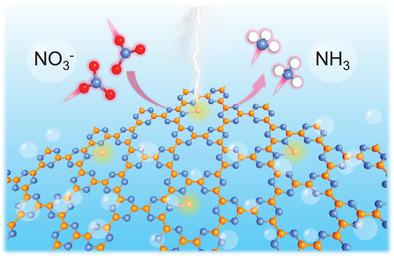
Selective nitrate‐to‐ammonia electrochemical conversion is an efficient pathway to solve the pollution of nitrate and an attractive strategy for low‐temperature ammonia synthesis. However, current studies for nitrate electroreduction (NO3RR) mainly focus on metal‐based catalysts, which remains challenging because of the poor understanding of the catalytic mechanism. Herein, taking single transition metal atom supported on graphitic carbon nitrides (g‐CN) as an example, the NO3RR feasibility of single‐atom catalysts (SACs) is first demonstrated by using density functional theory calculations. The results reveal that highly efficient NO3RR toward NH3 can be achieved on Ti/g‐CN and Zr/g‐CN with low limiting potentials of −0.39 and −0.41 V, respectively. Furthermore, the considerable energy barriers are observed during the formation of byproducts NO2, NO, N2O, and N2 on Ti/g‐CN and Zr/g‐CN, guaranteeing their high selectivity. This work provides a new route for the application of SACs and paves the way to the development of NO3RR.
中文翻译:
